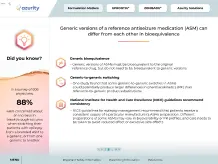
Did you know?
In a survey of 606 physicians,
88%
were concerned about an increase in breakthrough seizures when switching their patients with epilepsy from a branded ASM to a generic, or from one generic to another13

Generic versions of a reference antiseizure medication (ASM) can differ from each other in bioequivalence10,11

Generic bioequivalence
- Generic versions of ASMs must be bioequivalent to the original reference drug, but do not need to be bioequivalent to other generic versions10,11

Generic-to-generic switching
- One study found that some generic-to-generic switches in ASMs could potentially produce larger differences in pharmacokinetics (PK) than reference-to-generic product substitutions10

National Institute for Health and Care Excellence (NICE) guidelines recommend consistency
- NICE guidelines for epilepsy management recommend that patients receive a consistent supply of a particular manufacturer’s ASMs preparation. Different preparations of some ASMs may vary in bioavailability or PK profiles, and care needs to be taken to avoid reduced effect or excessive side effects12