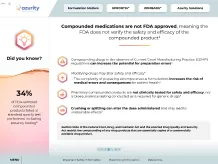
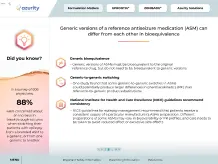
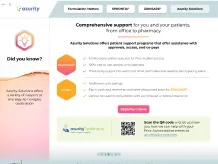
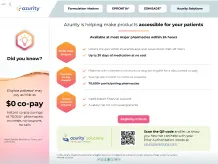
Did you know?
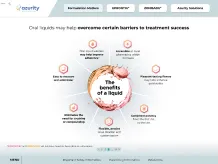
34%
(10/29) of FDA-sampled compounded products failed ≥1 standard quality test performed, including potency testing in 9 of 10 sampled compounds8

Compounded medications are not FDA approved, meaning the FDA does not verify the safety and efficacy of the compounded product4

Compounding drugs in the absence of Current Good Manufacturing Practice (CGMP) regulations can increase the potential for preparation errors5

Modifying drugs may alter safety and efficacy4,6
- The complexity of preparing extemporaneous formulations increases the risk of medical errors and consequences for patient health5

Pharmacy-compounded products are not clinically tested for safety and efficacy, nor is bioequivalence testing conducted as is required for generic drugs5

Crushing or splitting can alter the dose administered and may lead to undesirable effects7
Section 503A of the Federal Food, Drug, and Cosmetic Act and the enacted Drug Quality and Security Act restrict the compounding of any drug products that are essentially copies of a commercially available drug product.9